Purpose Of Serial Dilution Method
Doing this several times results in a range of concentrations The initial concentration and target range needed for a given assay determines the size and number of dilution steps required.. With molar concentrations it is safe to assume that the solute is evenly dispersed through the solution such that the concentrations change predictably with each dilution.. 16-fold (10 0 5-fold) dilution is called a half-logarithmic dilution or half-log dilution, and a 1.. These include A ten-fold serial dilution could be 1 M, 0 1 M, 0 01 M, 0 001 M Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale.. Often, serial dilutions are performed in steps of 10 or 100 They are described as ratios of the original and final concentrations. Wd Raid Manager Mac Download
purpose of dilution method
Doing this several times results in a range of concentrations The initial concentration and target range needed for a given assay determines the size and number of dilution steps required.. With molar concentrations it is safe to assume that the solute is evenly dispersed through the solution such that the concentrations change predictably with each dilution.. 16-fold (10 0 5-fold) dilution is called a half-logarithmic dilution or half-log dilution, and a 1.. These include A ten-fold serial dilution could be 1 M, 0 1 M, 0 01 M, 0 001 M Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale.. Often, serial dilutions are performed in steps of 10 or 100 They are described as ratios of the original and final concentrations. 518b7cbc7d Wd Raid Manager Mac Download
what is dilution method
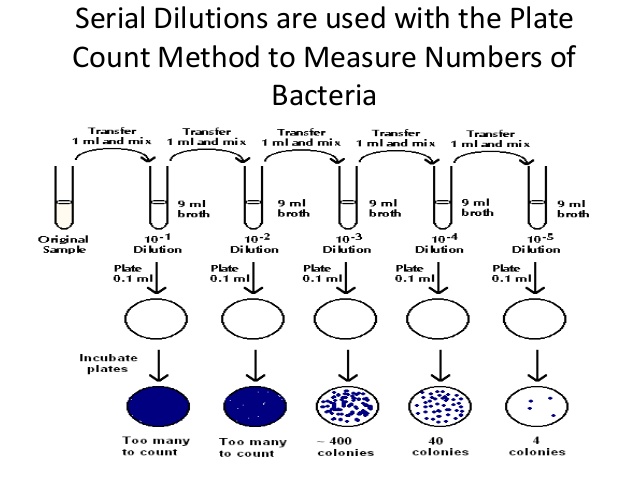
purpose of dilution method, does the dilution method work, what is dilution method, purpose of dilution technique, what is dilution technique Sketch 3 Download Mac
Mixing 100 µL of a stock solution with 900 µL of water makes a 1:10 dilution The final volume of the diluted sample is 1000 µL (1 mL) and the concentration is 1/10 that of the original solution. Download Mods Para Resident Evil 4 Pc